Research on TIM-3 and Alzheimer’s treatment is charting new territory in the quest for effective therapies against this debilitating disease. TIM-3, an immune checkpoint molecule, has emerged as a significant factor influencing how the brain’s immune cells, known as microglia, combat the accumulation of amyloid plaques associated with Alzheimer’s. By inhibiting TIM-3 function, studies suggest that microglia could be unleashed to clear these toxic plaques, potentially leading to improvements in cognitive function. As researchers delve into this promising approach, the intersection of Alzheimer’s disease research and immune system cancer therapy is becoming increasingly evident. This innovative strategy not only opens a pathway for better understanding the role of microglia function in Alzheimer’s but also paves the way for potential treatments involving anti-TIM-3 therapy.
Exploring the impacts of TIM-3 in Alzheimer’s interventions signifies a groundbreaking avenue in neurodegenerative disorders. This immune checkpoint molecule plays a pivotal role in regulating the brain’s immune response, particularly in how microglia address amyloid beta accumulation—an imposing feature in Alzheimer’s pathology. Studies indicate that targeting TIM-3 could facilitate the clearance of plaques, providing a novel strategy for cognitive function enhancement. With recent advancements in Alzheimer’s disease research paralleling techniques seen in immune system cancer therapies, there is a burgeoning optimism for therapeutic breakthroughs. As scientists continue to investigate anti-TIM-3 therapy, the potential for redefining treatment paradigms in Alzheimer’s becomes increasingly promising.
The Role of TIM-3 in Alzheimer’s Disease
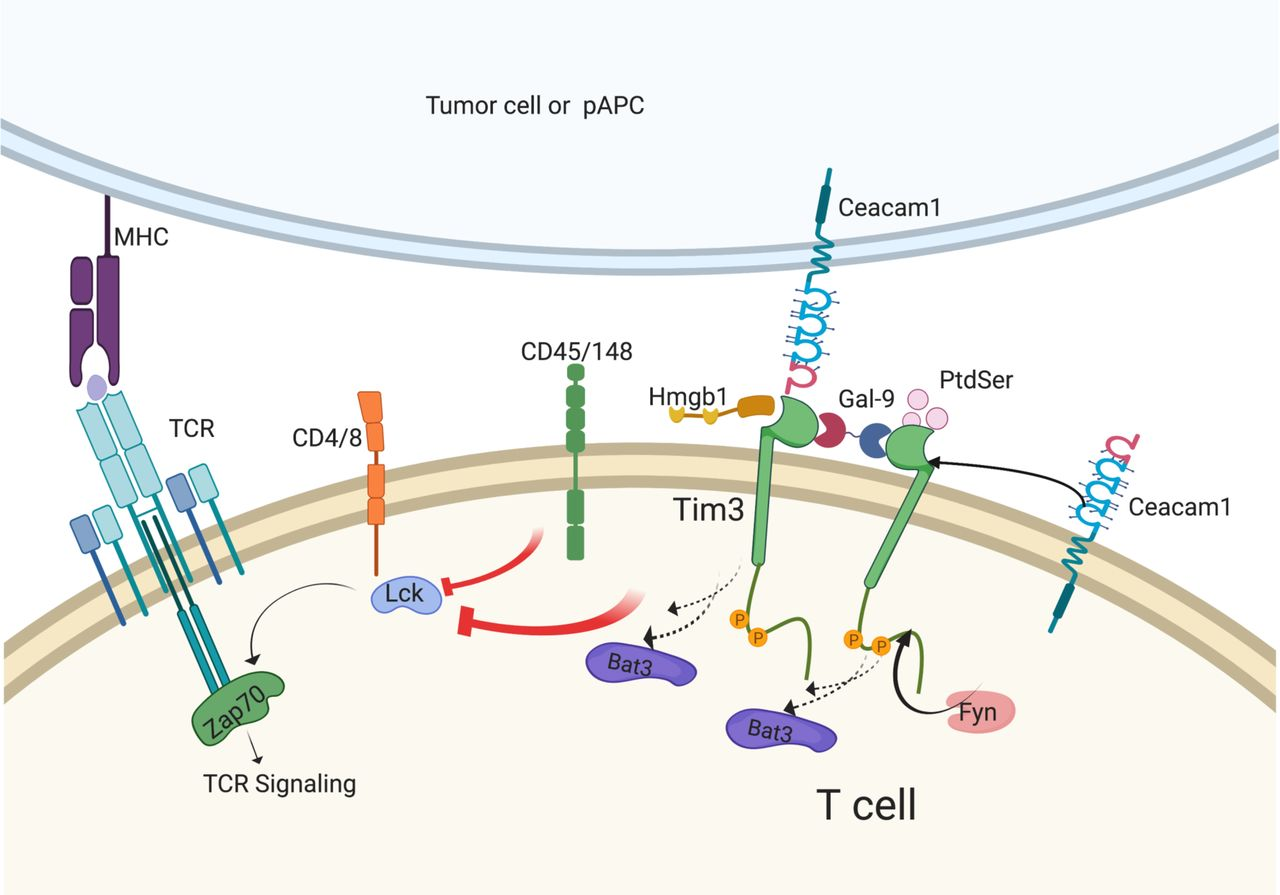
The TIM-3 molecule has garnered attention in recent Alzheimer’s disease research due to its role as a checkpoint molecule in the immune response. In the context of Alzheimer’s, TIM-3 inhibits microglia from effectively clearing amyloid plaques, which contribute to the disease’s cognitive decline. This relationship suggests that targeting TIM-3 may help restore the brain’s immune function by unblocking microglial activity, thereby improving cognitive function and potentially altering the course of Alzheimer’s.
Recent studies emphasize the significance of TIM-3 as a genetic risk factor associated with late-onset Alzheimer’s disease. The expression levels of TIM-3 are markedly higher in microglia of Alzheimer’s patients, suggesting that inhibiting this molecule could shift the immune cells from a homeostatic state to a more active role, capable of clearing amyloid plaques. By employing therapies that target TIM-3, there’s a potential pathway to enhance microglia function and mitigate the neurodegenerative processes of Alzheimer’s.
Microglia Function and Alzheimer’s Pathology
Microglia serve as the primary immune cells within the brain, playing crucial roles in various functions including synaptic pruning during development and immune defense against pathogens. However, in Alzheimer’s disease, microglia become dysfunctional due to the overexpression of checkpoint molecules like TIM-3. This dysfunction leads to impaired clearance of amyloid beta plaques, which can exacerbate neuronal damage and cognitive deficits. Thus, understanding microglia function is essential in directing new avenues of Alzheimer’s treatment.
The pathological transformation of microglia during the progression of Alzheimer’s disease indicates a need for therapeutic strategies that can revitalize their function. Research into anti-TIM-3 therapies suggests that these interventions could reactivate microglial clearance mechanisms and restore synaptic health. By leveraging insights into microglia’s dual role in neuroinflammation and plaque clearance, we can better formulate treatments that improve cognitive outcomes for those affected by Alzheimer’s.
Implications of Anti-TIM-3 Therapy
Exploring anti-TIM-3 therapy’s potential effects presents exciting prospects for Alzheimer’s disease management. Given that this therapy has shown promise in oncology for disrupting cancer cell immune evasion, repurposing similar strategies could strategically enhance microglial activity in Alzheimer’s. This could mean significant improvements in cognitive functions for Alzheimer’s patients, as microglia would be better equipped to handle the buildup of amyloid plaques that correlate with dementia.
As evidenced in preclinical mouse models, deleting the TIM-3 gene led to noticeable cognitive recovery as assessed through maze navigation tests. This directly correlates the inhibition of TIM-3 with enhanced memory performance, suggesting that anti-TIM-3 treatments could similarly facilitate cognitive function improvements in humans suffering from Alzheimer’s. Consequently, further research into these therapies could pave the way for innovative treatments that focus on immune modulation in neurodegenerative diseases.
Innovations in Alzheimer’s Research
The landscape of Alzheimer’s disease research is evolving, driven by discoveries like those concerning TIM-3. The integration of immune strategies previously restricted to cancer treatment now finds relevance in neurodegenerative disorders, creating opportunities for groundbreaking therapies. Continued innovation in this field may not only enhance early detection and intervention methods but may also optimize existing treatment protocols that focus on the immunological aspects of Alzheimer’s.
Ultimately, as the understanding of the immune system’s role in Alzheimer’s disease deepens, new therapeutic horizons are emerging. Strategies that harness the brain’s innate immune capabilities, such as anti-TIM-3 therapy, challenge long-standing paradigms in Alzheimer’s research. The goal is to develop multifaceted treatment strategies that not only target amyloid plaques but also invigorate neuroinflammatory responses conducive to cognitive health.
Challenges in Alzheimer’s Treatment Strategies
Despite advancements in Alzheimer’s treatment, numerous challenges persist, often stemming from the complexities of brain immunology. One significant barrier has been the difficulty in delivering therapies effectively across the blood-brain barrier. Anti-TIM-3 therapies could help to mitigate this issue by promoting microglial activity without risking the vascular integrity that current treatments often threaten.
Moreover, the diverse genetic landscape highlighted by factors like TIM-3 polymorphisms necessitates a more personalized approach to treatment. A deeper understanding of individual responses to therapies aimed at checkpoint molecules can aid in identifying which patient populations will benefit most from specific interventions. As research progresses, tailoring treatments to account for genetic risk factors like those linked with TIM-3 will be vital for success in overcoming Alzheimer’s.
Future Directions in Alzheimer’s Therapy
The future of Alzheimer’s therapy is poised to be revolutionized by strategies that think beyond traditional amyloid-targeting drugs. Anti-TIM-3 therapies represent a novel approach that combines neuroimmune regulation with cognitive health enhancement. As clinical trials evolve, the outcomes of such studies will be pivotal in determining the efficacy of breaking down pathological barriers and improving patient quality of life through immune-based strategies.
Moreover, interdisciplinary collaboration in Alzheimer’s research will be essential to maximize the potential of promising therapies like those targeting TIM-3. By bridging gaps between immunology and neurology, researchers and clinicians can cultivate a more comprehensive understanding of Alzheimer’s disease mechanisms. Such a synergistic approach will enhance the likelihood of discovering meaningful interventions that advance treatment paradigms in the battle against Alzheimer’s.
The Genetics of TIM-3 in Alzheimer’s Disease
The genetic underpinnings of TIM-3 are crucial in understanding its role in Alzheimer’s disease. Variants in the HAVCR2 gene, which encodes TIM-3, are associated with higher susceptibility to late-onset Alzheimer’s, highlighting the interplay between genetics and immune responses in the disease’s progression. By unraveling these genetic links, researchers can identify at-risk populations earlier and tailor interventions accordingly.
Research into TIM-3 polymorphisms might also provide insight into how different individuals respond to anti-TIM-3 therapies. With genetic profiling becoming increasingly common in medicine, harnessing the information around TIM-3 can guide therapeutic decisions and enable personalized medicine approaches in Alzheimer’s treatment, ensuring a more effective response tailored to the patient’s unique genetic profile.
Maximizing Microglial Potential Against Alzheimer’s
Understanding microglial function is critical for maximizing their potential in combating Alzheimer’s disease. These immune cells possess the capability to not only clear amyloid plaques but also modulate inflammatory processes within the brain. Unlocking the therapeutic potential of microglia through anti-TIM-3 therapy could significantly enhance their innate ability to restore homeostasis and promote cognitive function amidst Alzheimer’s pathology.
Future therapies will likely focus on strategies that empower microglia without triggering its over-restrictive mechanisms, such as those mediated by TIM-3. Encouraging active participation of microglia in synaptic health and plaque removal raises hope for developing treatments that could effectively reverse cognitive decline in Alzheimer’s patients, thus improving their overall quality of life.
The Intersection of Cancer and Neurodegenerative Research
The crossover between cancer therapy and neurodegenerative research, particularly concerning TIM-3, unveils a promising frontier for Alzheimer’s treatments. This intersection allows insights gained from immuno-oncology to be reapplied in a new context, potentially uncovering innovative strategies that utilize the body’s immune system to combat neurodegeneration. The exploration of immune checkpoint molecules, traditionally associated with tumor suppression, can therefore reshape Alzheimer’s therapeutic paradigms.
By leveraging the complex relationships between immune responses, cancer mechanisms, and Alzheimer’s disease progression, researchers can forge novel treatment pathways. This multidisciplinary approach not only enhances the understanding of Alzheimer’s but also brings forth potential therapies that could address both conditions, solidifying the significance of TIM-3 as a target for future interventions.
Frequently Asked Questions
What is TIM-3 and how does it relate to Alzheimer’s treatment?
TIM-3 (T-cell immunoglobulin and mucin domain 3) is a checkpoint molecule involved in regulating the immune response. In Alzheimer’s treatment, inhibiting TIM-3 may enhance the ability of microglia, the brain’s immune cells, to clear amyloid plaques associated with Alzheimer’s disease, potentially improving cognitive function.
How does TIM-3 impact microglia function in Alzheimer’s disease?
In Alzheimer’s disease, TIM-3 inhibits microglia from effectively clearing amyloid-beta plaques. By blocking TIM-3, microglia are freed to engage in phagocytosis, thus promoting the clearance of plaques and potentially leading to improved cognitive function in patients.
Can anti-TIM-3 therapy improve cognitive function in Alzheimer’s patients?
Preclinical studies indicate that anti-TIM-3 therapy can enhance microglial activity, resulting in the clearing of amyloid plaques. This therapeutic strategy may lead to significant cognitive function improvement by alleviating the cognitive deficits typically associated with Alzheimer’s disease.
What research supports the use of TIM-3 in Alzheimer’s disease treatment?
Recent studies published in Nature demonstrate that deleting TIM-3 in mouse models of Alzheimer’s allows microglia to attack and clear plaques, leading to measurable improvements in memory. This suggests that TIM-3 could be a promising target for future Alzheimer’s treatments.
How does TIM-3 deletion affect plaque accumulation in Alzheimer’s research models?
Deleting the TIM-3 gene in Alzheimer’s models significantly reduces plaque accumulation by enabling microglia to clear debris effectively. This not only reduces the quantity of plaques but also alters their composition, contributing to better cognitive performance in the affected mice.
What is the significance of TIM-3 as a genetic risk factor for Alzheimer’s disease?
TIM-3 has been identified as a genetic risk factor for late-onset Alzheimer’s disease. Genetic variations in the TIM-3 gene (HAVCR2) are linked to higher levels of this checkpoint molecule, which inhibits microglial function and contributes to plaque accumulation in the brain.
Could existing cancer therapies targeting TIM-3 be repurposed for Alzheimer’s treatment?
Yes, existing anti-TIM-3 therapies developed for cancer treatment could potentially be repurposed to target Alzheimer’s disease. By leveraging their ability to modulate the immune response, these therapies might aid in reducing plaque levels and improving cognitive function in Alzheimer’s patients.
How long has research into TIM-3 and Alzheimer’s treatment been conducted?
Research into TIM-3’s role in Alzheimer’s treatment has been ongoing for five years, involving extensive experimentation to understand how manipulating TIM-3 can influence plaque clearance and cognitive outcomes in Alzheimer’s disease models.
What are the next steps in TIM-3 Alzheimer’s research?
Future steps involve testing human anti-TIM-3 antibodies in mouse models that mimic Alzheimer’s to evaluate their effectiveness in halting plaque development and improving cognitive function, paving the way for potential clinical applications.
| Key Point | Details |
|---|---|
| Overview of TIM-3 | TIM-3 is an immune checkpoint molecule linked to late-onset Alzheimer’s, inhibiting microglial clearance of amyloid plaques. |
| Research Methods | Use of genetically modified mice lacking TIM-3 to observe improved clearance of plaques and cognitive functions. |
| Key Findings | Deletion of TIM-3 enhances microglial activity, leading to better memory performance and plaque clearance. |
| Implications for Treatment | Potential for anti-TIM-3 therapy to improve cognitive function in Alzheimer’s patients. |
| Future Directions | Ongoing research to test human anti-TIM-3 either directly for plaque reduction or cognitive improvement. |
Summary
TIM-3 and Alzheimer’s treatment represent a promising frontier in the battle against Alzheimer’s disease. Recent research has demonstrated that targeting the TIM-3 checkpoint molecule can unleash microglial cells to clear harmful plaques from the brain, potentially restoring cognitive function. By utilizing existing anti-TIM-3 therapies, researchers aim to translate these findings into effective treatments for Alzheimer’s, a disease affecting a significant portion of the elderly population. As studies continue to unfold, the hope is to identify viable approaches that may not only improve memory but also alter the disease’s progression.