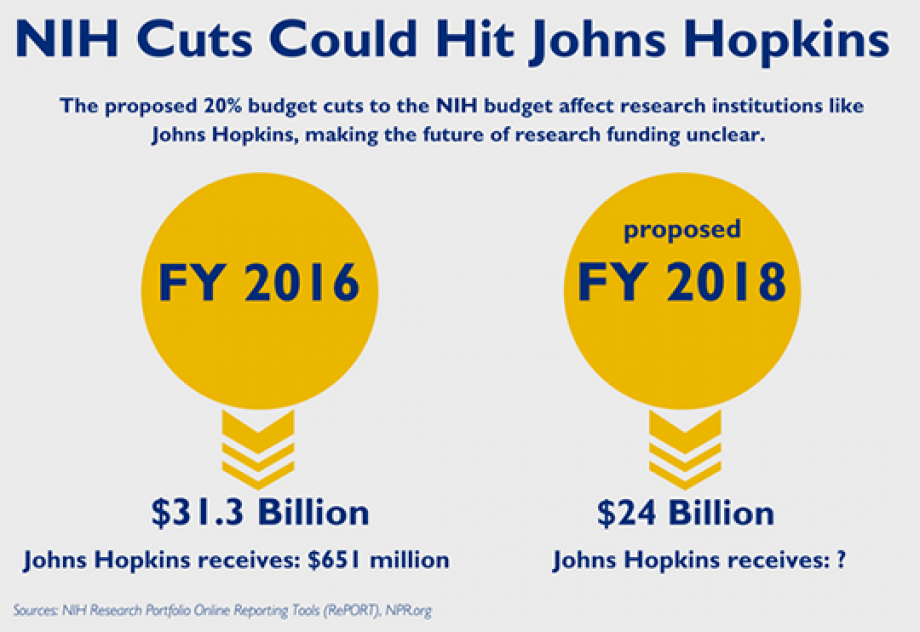
Funding cuts in medical research pose a significant threat to the integrity and effectiveness of clinical studies aimed at enhancing patient safety in medical research. As federal research grants dwindle, the ability of institutions like Harvard to uphold necessary IRB oversight is severely compromised, leading to a rippling effect across the entire research landscape. Such cuts undermine the impact of funding on research, jeopardizing not only ongoing projects but also future innovations that could save lives. When patient safety is put at risk, ethical considerations become paramount, making it imperative to understand the NIH funding effects on healthcare advancements. Ultimately, a decline in resources leads to a deterioration in the ethical standards and protective measures that are crucial for ensuring participant welfare in research.
Recent reductions in financial support for scientific inquiry threaten the progress and safety measures essential for medical trials. The cancellation of vital funding streams disrupts oversight mechanisms that ensure ethical practices and protection for individuals involved in clinical studies. As institutions grapple with how to navigate the limited resources, the fundamental principle of patient care can easily become overshadowed by bureaucratic challenges. The implications of such budget constraints extend beyond the university walls, affecting public trust and the operational integrity of research programs across the nation. It is vital that we address the challenges faced by research institutions to foster an environment that prioritizes both innovation and the well-being of research participants.
The Impact of Funding Cuts in Medical Research
Funding cuts in medical research can have severe repercussions on the safety and welfare of patients involved in clinical trials. One of the more significant ways these cuts affect patient safety is through the disruption of Institutional Review Boards (IRBs), which are fundamental in overseeing research protocols and ensuring that ethical standards are maintained. When financial resources dwindle, IRB operations may be hampered, leading to lapses in oversight. This creates a risk for participants who may be exposed to insufficiently reviewed research, increasing the chances of unforeseen adverse effects during clinical trials.
Moreover, the halting of funding not only impacts existing studies but also deters potential new research projects aimed at improving patient outcomes. As federal grant opportunities diminish, many institutions are forced to scale back or completely abandon initiatives designed to enhance patient safety. This is particularly concerning considering that the public’s trust in medical research is partly dependent on the rigorous ethical standards enforced during clinical trials — standards that are maintained predominantly through well-funded and active IRBs.
The Role of IRB Oversight in Safeguarding Participants
Institutional Review Boards (IRBs) function as a critical component of safeguarding the rights and welfare of participants in medical research. They meticulously evaluate research proposals, assessing potential risks and determining whether the benefits of the study align with the ethical principles of respect, beneficence, and justice. The review process is designed to ensure informed consent is appropriately obtained and maintained throughout the duration of the study, which is vital for preserving patient autonomy. With the disruptions caused by funding cuts, the ability of IRBs to conduct thorough reviews and provide ongoing oversight is jeopardized, thereby compromising patient safety.
Furthermore, IRBs also play an essential role in educating researchers about regulatory compliance and ethical research practices. When funding is limited, training resources may be cut, leaving researchers ill-prepared to navigate the ethical complexities inherent in medical studies. This gap can lead to oversights that ultimately endanger patients, as researchers may inadvertently neglect the necessary protocols to protect study subjects. Hence, the continuity of funding for medical research is directly linked to the integrity and effectiveness of IRB oversight, which is paramount for the safety of all clinical research participants.
Understanding NIH Funding Effects on Patient Safety
The National Institutes of Health (NIH) provides crucial funding that underpins a vast array of clinical research initiatives aimed at enhancing patient safety and treatment outcomes. By supporting research that complies with rigorous ethical standards, NIH funding directly contributes to the development of safe medical practices and innovative therapies. When NIH budgets are cut, the ripple effect can be devastating, as many promising studies may be put on hold, reducing the pace of scientific discovery and potentially delaying access to new treatments for patients.
Additionally, NIH funding is often used to facilitate compliance with ethical research norms, such as the requirement for Institutional Review Board oversight. When financial resources are restricted, institutions may struggle to allocate adequate support for IRB activities, directly affecting their capacity to monitor ongoing studies. This compromise in oversight not only places participants at risk but might also exacerbate existing disparities in healthcare research, as underfunded institutions may be less able to implement robust ethical safeguards compared to their better-funded counterparts.
Ethics in Medical Research: A Crucial Foundation
The ethics of medical research serve as the foundation upon which trust between researchers, participants, and the broader community is built. Research ethics encompass a set of principles designed to protect the dignity, rights, and welfare of research subjects, which is critical for fostering public confidence in medical advancements. The historical missteps in research ethics, including notorious studies like Tuskegee and Willowbrook, highlight the necessity of having strong ethical oversight in place, something that is often supported by government funding.
With funding constraints, the capacity to uphold ethical standards diminishes, potentially leading to research practices that prioritize outcomes over participant safety and rights. The ethical oversight provided by IRBs relies not only on committed professionals but also on appropriate financing. Hence, ensuring that ethical guidelines are maintained in medical research is a complex interplay of funding, oversight, and rigorous training — all essential for protecting participants in clinical studies.
Patient Safety: Central to Research Integrity
Patient safety is paramount in clinical research and reflects the broader integrity of the research process. Research initiatives that put safety first often see better participant compliance, more reliable data outcomes, and ultimately, a greater overall impact on public health. The discipline of patient safety is embedded in careful study design, adherence to ethical guidelines, and consistent monitoring by IRBs, which are in turn funded through federal grants. The reliance on a solid funding base empowers institutions to uphold high standards in patient safety across the research landscape.
When funding cuts threaten this framework, the integrity of patient safety practices in research is jeopardized. Uncertainties about funding lead to hesitancy in conducting thorough safety evaluations and may result in rushed studies lacking adequate oversight. As a result, the ramifications can be significant, potentially impacting the efficacy of treatments being researched and skewing the critical data that informs future clinical practices. Thus, a consistent and robust funding stream is vital for ensuring that patient safety remains at the forefront of medical research.
Collaborative Research: Overcoming Funding Barriers
Collaboration across multiple research facilities can lead to significant advancements in medical research, particularly in developing therapies that require diverse expertise and resources. The SMART IRB system exemplifies this collaborative approach, efficiently facilitating oversight for studies involving numerous sites. However, with the recent funding cuts, many collaborative efforts are stalling, undermining the progress that has been made in cooperative research. The inability to secure needed financial support can lead to significant delays in critical research, thereby affecting the timelines for bringing new treatment options to patients.
Additionally, the cancellation of grants has often forced institutions to be more selective about the studies they pursue, potentially ignoring projects that hold promise for patient safety improvements simply because they lack immediate funding. This short-sighted approach not only hampers the personal development of researchers but also dilutes the collective effort to innovate in ways that directly benefit patient care. The need for collaborative initiatives in medical research is clear; hence, securing funding must be viewed as an investment in the future of healthcare.
Building Public Trust Through Research Oversight
Building public trust in medical research is essential for the successful recruitment of participants and the overall efficacy of clinical trials. When individuals express skepticism towards the research process — often due to previous ethical violations — it becomes challenging to find volunteers willing to participate in potentially life-changing studies. Establishing a robust oversight mechanism through consecutive funding is critical in ensuring that ethical guidelines are consistently followed, which in turn reassures the public of their safety and the integrity of the research process.
The funding cuts can erode this trust, as stakeholders may perceive a lack of commitment to ethical practices in research. As studies face interruptions or are forced to adopt subpar safety measures due to budget constraints, the message sent to the community is one of diminished accountability. To restore confidence in medical research, robust oversight through well-funded IRBs and ethical governance must be a priority, ensuring participants feel safe and valued as contributors to knowledge advancement.
Addressing Historical Ethical Concerns in Research
The history of medical research is punctuated with instances of ethical failures that have led to severe violations of trust and patient rights. It is essential to learn from past atrocities, such as the Tuskegee Syphilis Study and the controversial research conducted at Willowbrook, to ensure they are never repeated. The establishment of IRBs and the implementation of strict ethical guidelines were instituted primarily to prevent such violations and uphold the dignity of research participants. However, these systems are fundamentally dependent on a stable framework of funding.
In light of recent funding cuts, there is a genuine concern that the lessons learned from historical transgressions in research ethics may be overlooked or diminished. Reduced funding compromises the training of IRB members and diminishes the capacity for meticulous oversight, thus putting research participants at risk. We must commit to ensuring that historical ethical concerns are comprehensively addressed in present-day research through adequate support and funding, fortifying the integrity and safeguards necessary for ethically sound medical research.
Future Directions: Ensuring Sustainable Funding for Research
Sustainable funding for medical research is increasingly vital in maintaining the safety and rights of research participants. As the landscape of funding evolves, it is crucial for researchers and institutions to advocate for greater investment in ethics and oversight mechanisms. By rallying support for research initiatives that prioritize ethical standards, we can ensure that the safety of patients remains a top priority and that proper IRB oversight continues to protect their rights throughout the research process.
Looking forward, developing diversified funding strategies, such as public-private partnerships and grants aimed specifically at ethical oversight, can create a more resilient framework for medical research funding. Such strategies not only protect researchers from financial volatility but also promote public confidence in the transparency and integrity of clinical trials. Together, these efforts can foster an environment where patient safety is embedded in the research agenda, ultimately leading to more responsible scientific advancement.
Frequently Asked Questions
How do funding cuts in medical research affect patient safety?
Funding cuts in medical research, such as those imposed by the Trump administration, can significantly impact patient safety. When research funding is interrupted, oversight mechanisms like Institutional Review Boards (IRBs) struggle to function effectively. These disruptions may lead to delays in research, preventing necessary studies from proceeding and jeopardizing the safety of participants involved in clinical trials.
What is the impact of funding cuts on IRB oversight in medical research?
Funding cuts in medical research greatly undermine the capacity of Institutional Review Boards (IRBs) to carry out their essential oversight functions. Without adequate funding, IRBs may face staffing shortages and resource limitations, which can hinder their ability to thoroughly review research proposals, ensure compliance with ethical standards, and ultimately protect the rights and welfare of research participants.
How do NIH funding effects shape medical research ethics?
NIH funding plays a critical role in upholding medical research ethics by providing the necessary resources for comprehensive oversight, including the work of IRBs. When funding is cut, the ethical standards that guide research practices may be compromised, risking the integrity of research studies and undermining public trust in medical research.
What are the consequences of reduced medical research funding on patient rights?
Reduced medical research funding can lead to significant consequences for patient rights. With less financial support, studies may be halted, IRB reviews delayed, and the overall quality of oversight diminished. This creates an environment where patient rights and safety may not be adequately safeguarded, leading to potential ethical violations.
What role does federal funding play in ensuring the safety of participants in medical research?
Federal funding is crucial in ensuring participant safety in medical research, as it provides resources for the necessary oversight by IRBs and supports the administrative costs associated with ethical reviews. Cuts to federal funding can disrupt this system, potentially leading to lapses in the protection of patient rights and safety.
How can the impact of funding cuts on research collaboration be assessed?
The impact of funding cuts on research collaboration can be assessed by examining the reduction in the number of active studies and the ability of multiple sites to work together on projects. When funding is cut, collaborative efforts like those facilitated by SMART IRB suffer, leading to delays in research and potentially hindering advancements in medical treatments.
In what ways does halting funding disrupt medical research?
Halting funding disrupts medical research by freezing ongoing studies, preventing new clinical sites from joining, and leading to the cancellation of research contracts. This ultimately hampers the ability to ensure patient safety, as studies cannot progress, and necessary medical innovations may be delayed indefinitely.
| Key Point | Details |
|---|---|
| Impact of Funding Cuts | The Trump administration’s freeze of over $2 billion in federal research grants halts efforts to ensure patient rights and safety in medical studies. |
| Role of NIH Funding | NIH funding supports Institutional Review Boards (IRBs) that ensure compliance with laws and protect the rights and welfare of research participants. |
| Function of IRBs | IRBs review research proposals, manage consent processes, assess risks, and maintain oversight to protect study participants. |
| Historical Context | The establishment of IRBs arose from historical abuses in medical research, emphasizing the need for ethical oversight. |
| Consequences of Funding Gaps | Cuts to funding can halt research midstream, risking harm to participants and undermining public trust in medical research. |
Summary
Funding cuts in medical research have profound impacts on the safety and rights of participants. Recent freezes in federal funding disrupt established systems like IRBs that ensure compliance and ethical standards in research. Without these vital resources, the protection of patients is compromised, possibly leading to public mistrust and significant setbacks in medical advancements. It is essential for the community to advocate for sustained funding to safeguard participant welfare and foster innovation in medical research.